Published online Mar 16, 2023. doi: 10.4253/wjge.v15.i3.114
Peer-review started: September 23, 2022
First decision: November 4, 2022
Revised: December 17, 2022
Accepted: February 10, 2023
Article in press: February 10, 2023
Published online: March 16, 2023
Processing time: 174 Days and 5.9 Hours
Gastric cancer is the fifth most common cancer and in 2018, it was the third most common cause of cancer-related deaths worldwide. Endoscopic advances con
Core Tip: Consider screening for gastric cancer in appropriate patient populations, as early gastric cancer outcomes are associated with improved survival. Use of different imaging modalities during endoscopy such as narrow-band imaging may improve detection of gastric cancer and premalignant gastric conditions. Endoscopic mucosal resection and submucosal dissection have shown favorable long-term outcomes. While there are no established evidence-based gastric cancer surveillance guidelines in the United States, other studies have suggested annual surveillance after gastric cancer resection. Endoscopic surveillance of premalignant gastric conditions may be considered, with closer intervals in patients with evidence of dysplasia.
- Citation: Park E, Nishimura M, Simoes P. Endoscopic advances in the management of gastric cancer and premalignant gastric conditions. World J Gastrointest Endosc 2023; 15(3): 114-121
- URL: https://www.wjgnet.com/1948-5190/full/v15/i3/114.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i3.114
Globally, gastric cancer is the fifth most common cancer, with an estimated 1 million new cases annually[1-3]. In 2020, it was the fourth most common cause of cancer-related deaths worldwide[1-3]. In recent years, many endoscopic advances have been made for both the diagnosis and therapy of gastric cancer. In this article, we review the endoscopic tools used for the diagnosis and treatment of gastric cancer and premalignant gastric conditions. First, we discuss briefly the epidemiology of gastric cancer and outline the differences in outcomes for early-stage vs late-stage gastric cancer. We then review the endoscopic approaches in the diagnosis and treatment of gastric cancer and premalignant conditions. Finally, we review the current guidelines for endoscopic surveillance of gastric cancer and premalignant conditions.
According to the International Agency for Research on Cancer GLOBOCAN project, there were over 1 million new cases of gastric cancer and 768793 deaths from gastric cancer in 2020[4]. The majority of cases of gastric cancer are found in East Asia such as Japan and Korea, as well as Eastern Europe and South America. It is also more highly associated with the male sex, as well as with increasing age[1]. Other risk factors include low socio-economic status, cigarette smoking, alcohol use, pernicious anemia, and autoimmune gastritis[5]. Obesity and gastroesophageal reflux disease are associated with an increased risk of specifically gastric cardia cancer[2].
Infection with Helicobacter pylori (H. pylori) is a significant cause of gastric cancer[6]. Chronic infection may lead to inflammatory mucosal changes including atrophic gastritis and ultimately intestinal metaplasia[7]. The risk of gastric cancer in patients with H. pylori may also be increased with salted food intake, as there is thought to be a synergistic effect. Treatment of H. pylori may reduce the risk of the development of gastric cancer, and earlier treatment of this infection has been associated with risk reduction of gastric cancer[1].
Overall, outcomes for gastric cancer are poor, especially in advanced stages[7,8]. Correa’s cascade describes the development from atrophic gastritis to intestinal metaplasia, dysplasia, and ultimately invasive gastric adenocarcinoma[9]. Each step in this cascade offers an opportunity to screen and perform surveillance in order to arrest the development of gastric cancer. The implementation of gastric cancer screening programs in countries with a high incidence of gastric cancer such as Japan and South Korea has demonstrated that earlier diagnosis leads to improved survival[7]. The biennial screening program in South Korea has led to an increase in the diagnosis of early gastric cancer from 39% to 73%, as well as an increase in 5-year survival from 46% to 75%[6]. The difference in survival between early and late stage gastric cancer emphasizes the importance of early diagnosis and treatment.
Conventional endoscopic evaluation of the stomach is performed with white-light endoscopy. When performing endoscopy with white-light imaging, it is recommended to take sufficient time to observe the stomach. Prior studies have demonstrated that endoscopists who took more time to observe the stomach closely detected a greater number of early gastric cancer lesions[10]. The use of defoaming agents such as simethicone to wash the stomach during evaluation may also improve visibility of the stomach lining[10]. The sensitivity of white-light endoscopy for detecting gastric cancer and premalignant lesions has been reported to be anywhere between 30%-70%[11-13]. Additionally, there are now suggested standard mapping protocols in place in order to carefully examine the entire gastric mucosa and ensure that no areas were not viewed under white-light endoscopy[14]. In recent years, other methods of endoscopic visualization have been developed in hopes of improving the diagnosis of gastric cancer.
In chromoendoscopy, indigo carmine or a similar stain is applied topically to the mucosa to help improve identification of gastric cancer or premalignant gastric lesions. Early prospective studies suggest that the use of chromoendoscopy aids in the diagnosis of gastric neoplasia compared to conventional endoscopy[15]. Prior meta-analysis of the diagnostic efficacy of chromoendoscopy suggests that there is an increased diagnostic efficacy and detection of early gastric cancer and premalignant gastric conditions, with a sensitivity of 90% and specificity of 82%[16]. However, it is important to note that no randomized controlled trials have yet been performed to evaluate chromoendoscopy.
In narrow-band imaging (NBI), wavelengths of light used for visualization are limited to a specific band. This allows for improved visualization of the architecture of the mucosa[14,17]. NBI is now used as part of a diagnostic algorithm known as magnifying endoscopy simple diagnostic algorithm for early gastric cancer for classifying early gastric cancer. With the use of NBI, the lesion is evaluated for a demarcation line (DL). If a DL is present, the lesion is then evaluated for an irregular microvascular pattern (IMVP) and an irregular micro surface pattern (IMSP). If the lesion has either an IMVP or IMSP, the diagnosis of early gastric cancer is made[18].
The data for NBI in the diagnostic efficacy and detection of gastric cancer and premalignant gastric lesions is variable. For gastric cancer specifically, there does not appear to be a significant difference in diagnostic yield[19]. However, prior studies indicate that NBI may improve detection of premalignant lesions such as intestinal metaplasia. One randomized controlled trial revealed that non-magnifying NBI had a significantly higher detection rate than white-light endoscopy in the diagnosis of intestinal metaplasia, but not gastric cancer[20]. In a systematic review of ten studies (eight prospective studies and two retrospective studies), NBI appeared to significantly increase the detection of intestinal metaplasia[21,22]. Use of NBI should thus be considered in high risk populations to evaluate for premalignant gastric lesions.
Confocal laser endomicroscopy is an endoscopic technique that uses a low-power laser to obtain very high magnification of the mucosal layer of the gastrointestinal tract. Prior studies including meta-analyses evaluating the diagnostic value of confocal laser endomicroscopy suggest that CLE provides high sensitivity and specificity for the diagnosis of gastric cancer[23,24]. CLE also appears to have a high sensitivity and specificity in the diagnosis of premalignant gastric lesions. A meta-analysis of four studies including 218 patients and 579 lesions evaluating CLE for the diagnosis of intestinal metaplasia showed a pooled sensitivity of 97% and specificity of 94%[23].
Flexible spectral imaging color enhancement (FICE) is another technique with the potential to detect early gastric cancer. In FICE, a narrow bandwidth is obtained from a white-light image without optical filters. This allows for the possible visualization of laminar structures and blood flow in the gastrointestinal mucosa that has been altered by inflammation or malignancy, which will appear as high contrast compared to normal mucosa[25]. Prior studies have suggested that FICE is helpful in distinguishing between non-neoplastic and neoplastic lesions of the stomach[26]. However, FICE is limited in visualization of the mucosal microvasculature of the tumor surface, and visualization may need to be supplemented with additional imaging techniques[25].
Artificial intelligence is a growing field in gastroenterology and has shown efficacy in the detection of many different gastrointestinal lesions. Initial studies of neural networks generated from endoscopic images under both white-light endoscopy and NBI show high sensitivity for both methods in detecting lesions[27,28]. Real-time artificial intelligence detection of gastric lesions has yet to be studied.
Endoscopic ultrasound (EUS) allows for assessment of the depth of gastric cancer as it is able to distinctly identify the layers of the stomach[29]. Ultrasound can be achieved using the linear or radial transducers on the endoscope or with a through-the-scope ultrasound catheter probe. The five layers of the gastric wall are identified by their alternating hyperechoic and hypoechoic appearance[29]. EUS therefore is utilized to determine the T category of staging according to the TNM classification. A query of the Surveillance, Epidemiology, and End Results-Medicare claims database performed in 2016 suggested that patients who underwent EUS were more likely to receive National Comprehensive Cancer Network recommended care such as perioperative chemotherapy[30]. Prior meta-analysis of 54 clinical studies suggests that EUS is successfully able to differentiate T1 and T2 stages from T3 and T4 stages, with reported sensitivity of 86% and specificity of 91%[31]. Specifically, EUS has been reported to distinguish T1 from more advanced stages with a sensitivity of 83% and specificity of 96%[31]. However, there was significant heterogeneity among the studies included, with some studies using older TNM classification systems. Factors that appear to decrease EUS accuracy include larger cancer diameter, ulceration, undifferentiated histology, and proximal location[31,32]. Further studies are needed to evaluate the staging accuracy of EUS based on the updated TNM classification system.
EUS is also a modality to help determine nodal involvement of gastric cancer. Larger size of the node, sharp margins, and hypoechoic pattern may help endosonographers determine lymph node invo
Prior studies have shown that patients diagnosed with early gastric cancer who did not undergo resection, whether endoscopic or surgical, had a greater 5-year risk for progression to the advanced stage[33,34]. Current guidelines established for the therapy of early gastric cancer recommend resection once the diagnosis has been established[33,34]. Traditional criteria for endoscopic resection of early gastric cancer included adenocarcinoma that was 2 cm or less in diameter without ulceration or lymph node or vascular involvement[35,36]. More recently, this criteria has been expanded as additional studies have shown favorable long term outcomes of endoscopic resection in early gastric cancer, especially with the advances made in endoscopic submucosal dissection (ESD)[35,36]. In fact, multiple studies have now found 5-year survival rates to be nearly 100%[34].
Endoscopic mucosal resection (EMR) is a procedure where a submucosal injection is used to lift the lesion, followed by resection of the lesion using snare. This technique allows for safe removal of intramucosal cancers that are 2 cm or less in diameter[37]. EMR has proven to be an effective treatment for early gastric cancer in terms of long-term outcomes. In one prior study in Japan with 479 cases of gastric cancer treated with EMR, there were no gastric cancer-related deaths during a median follow up period of 38 mo[38]. Notably, the rates of complete resection with EMR decrease with larger lesions with prior studies demonstrating complete resection rates as low as 20%-30% in lesions greater than 2 cm[39].
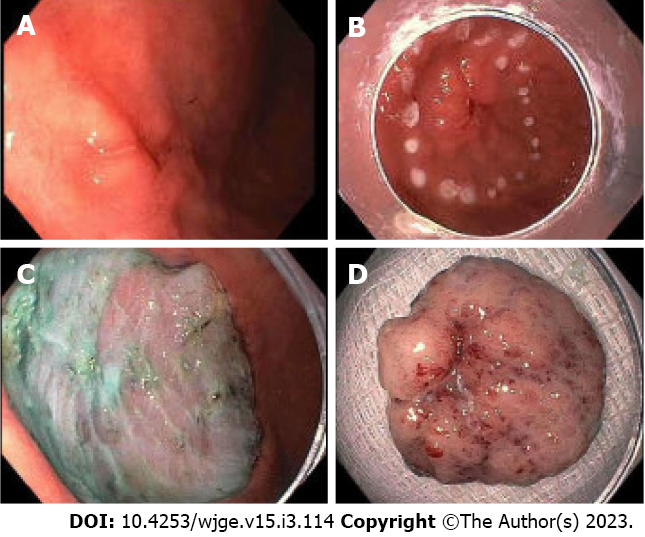
ESD is a technique in which the submucosal layer is injected to lift the lesion. Following injection, careful dissection of the submucosal layer from the muscular layer is performed using through-the-scope endoscopic knives, until the entire lesion is completely removed[40] (Figure 1). More recently, tools including various endoscopic knives and hemostatic forceps have been developed in order to perform quicker, more secure, and more precise incisions[38,39,41]. ESD has been shown to be more effective at complete resection of larger gastric cancer lesions[34]. In a meta-analysis of 18 observational studies, ESD proved to have a greater incidence of complete and curative resection compared to patients who underwent EMR[42]. ESD also has been associated with a lower risk of recurrence compared to EMR.
There are no randomized trials yet comparing endoscopic and surgical management of early gastric cancer, though several studies report favorable outcomes in endoscopic resection. Endoscopic resection has been associated with fewer complications and an improved quality of life when compared to surgical resection[43,44], likely because endoscopic resection allows for preservation of the stomach. Notably, studies also suggest that the recurrence rates are significantly higher with endoscopic resection than surgical resection[45].
A more recently developed technique for removal of early gastric cancer lesions is laparoscopic endoscopic cooperative surgery (LECS). LECS involves endoscopic mucosal or submucosal dissection with laparoscopic seromuscular resection, with the intention to preserve as much of the normal stomach as possible[45,46]. LECS was initially used for the removal of submucosal tumors, but more recently has been studied for the removal of early gastric cancer[47,48]. It is important to note that with all types of LECS, laparoscopic peri-gastric lymph node dissection is also performed[46].
At present, there are no established evidence-based gastric cancer surveillance guidelines in the United States. Patients with gastric cancer that was treated with resection continue to have a risk for metachronous gastric cancer. Prior studies report an incidence of metachronous gastric cancer of 3 to 4 percent per year[49]. Japanese guidelines suggest annual or biannual endoscopic surveillance. Other studies have recommended earlier follow-up of 3 mo after resection, followed by gradual spacing to 6 mo and then a year if no lesion identified[49].
Premalignant gastric conditions include atrophic gastritis and intestinal metaplasia. There are various guidelines for the surveillance of these premalignant conditions. The European Society of Gas
Prior studies report varying rates of progression of dysplastic lesions to gastric cancer, ranging anywhere from 0% to 73% per year[53]. This is in part due to the difference between specific popu
Over recent years, many endoscopic advances have been made for the diagnosis and treatment of gastric cancer lesions. Further studies to enhance visualization and diagnosis of early-stage gastric cancer tumors as well as different techniques for removal should be encouraged.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: American College of Gastroenterology; American Gastroenterological Association; American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao W, China; Miao Y, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2686] [Article Influence: 537.2] [Reference Citation Analysis (5)] |
| 2. | Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol. 2020;18:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 919] [Article Influence: 183.8] [Reference Citation Analysis (1)] |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 61628] [Article Influence: 15407.0] [Reference Citation Analysis (171)] |
| 4. | Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer, 2018. [cited 15 July 2022]. Available from: https://gco.iarc.fr/today/fact-sheets-cancers. |
| 5. | Kumar S, Metz DC, Ellenberg S, Kaplan DE, Goldberg DS. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology. 2020;158:527-536.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 6. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1986] [Article Influence: 248.3] [Reference Citation Analysis (0)] |
| 7. | Huang RJ, Hwang JH. Improving the Early Diagnosis of Gastric Cancer. Gastrointest Endosc Clin N Am. 2021;31:503-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Eusebi LH, Telese A, Marasco G, Bazzoli F, Zagari RM. Gastric cancer prevention strategies: A global perspective. J Gastroenterol Hepatol. 2020;35:1495-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 9. | Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 503] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 10. | Yao K, Uedo N, Kamada T, Hirasawa T, Nagahama T, Yoshinaga S, Oka M, Inoue K, Mabe K, Yao T, Yoshida M, Miyashiro I, Fujimoto K, Tajiri H. Guidelines for endoscopic diagnosis of early gastric cancer. Dig Endosc. 2020;32:663-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 11. | Young E, Philpott H, Singh R. Endoscopic diagnosis and treatment of gastric dysplasia and early cancer: Current evidence and what the future may hold. World J Gastroenterol. 2021;27:5126-5151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (3)] |
| 12. | Kim JH, Kim YJ, An J, Lee JJ, Cho JH, Kim KO, Chung JW, Kwon KA, Park DK, Kim JH. Endoscopic features suggesting gastric cancer in biopsy-proven gastric adenoma with high-grade neoplasia. World J Gastroenterol. 2014;20:12233-12240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Buxbaum JL, Hormozdi D, Dinis-Ribeiro M, Lane C, Dias-Silva D, Sahakian A, Jayaram P, Pimentel-Nunes P, Shue D, Pepper M, Cho D, Laine L. Narrow-band imaging versus white light versus mapping biopsy for gastric intestinal metaplasia: a prospective blinded trial. Gastrointest Endosc. 2017;86:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Rey JF, Lambert R; ESGE Quality Assurance Committee. ESGE recommendations for quality control in gastrointestinal endoscopy: guidelines for image documentation in upper and lower GI endoscopy. Endoscopy. 2001;33:901-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Sakai Y, Eto R, Kasanuki J, Kondo F, Kato K, Arai M, Suzuki T, Kobayashi M, Matsumura T, Bekku D, Ito K, Nakamoto S, Tanaka T, Yokosuka O. Chromoendoscopy with indigo carmine dye added to acetic acid in the diagnosis of gastric neoplasia: a prospective comparative study. Gastrointest Endosc. 2008;68:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Zhao Z, Yin Z, Wang S, Wang J, Bai B, Qiu Z, Zhao Q. Meta-analysis: The diagnostic efficacy of chromoendoscopy for early gastric cancer and premalignant gastric lesions. J Gastroenterol Hepatol. 2016;31:1539-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Barbeiro S, Libânio D, Castro R, Dinis-Ribeiro M, Pimentel-Nunes P. Narrow-Band Imaging: Clinical Application in Gastrointestinal Endoscopy. GE Port J Gastroenterol. 2018;26:40-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Muto M, Yao K, Kaise M, Kato M, Uedo N, Yagi K, Tajiri H. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc. 2016;28:379-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 19. | Kakushima N, Yoshida N, Doyama H, Yano T, Horimatsu T, Uedo N, Yamamoto Y, Kanzaki H, Hori S, Yao K, Oda I, Tanabe S, Yokoi C, Ohata K, Yoshimura K, Ishikawa H, Muto M. Near-focus magnification and second-generation narrow-band imaging for early gastric cancer in a randomized trial. J Gastroenterol. 2020;55:1127-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Ang TL, Pittayanon R, Lau JY, Rerknimitr R, Ho SH, Singh R, Kwek AB, Ang DS, Chiu PW, Luk S, Goh KL, Ong JP, Tan JY, Teo EK, Fock KM. A multicenter randomized comparison between high-definition white light endoscopy and narrow band imaging for detection of gastric lesions. Eur J Gastroenterol Hepatol. 2015;27:1473-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Uedo N, Ishihara R, Iishi H, Yamamoto S, Yamada T, Imanaka K, Takeuchi Y, Higashino K, Ishiguro S, Tatsuta M. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 263] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Desai M, Boregowda U, Srinivasan S, Kohli DR, Al Awadhi S, Murino A, Yu LHK, Dinis-Ribeiro DM, Sharma P. Narrow band imaging for detection of gastric intestinal metaplasia and dysplasia: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:2038-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Zhang HP, Yang S, Chen WH, Hu TT, Lin J. The diagnostic value of confocal laser endomicroscopy for gastric cancer and precancerous lesions among Asian population: a system review and meta-analysis. Scand J Gastroenterol. 2017;52:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Guo YT, Li YQ, Yu T, Zhang TG, Zhang JN, Liu H, Liu FG, Xie XJ, Zhu Q, Zhao YA. Diagnosis of gastric intestinal metaplasia with confocal laser endomicroscopy in vivo: a prospective study. Endoscopy. 2008;40:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Osawa H, Yamamoto H. Present and future status of flexible spectral imaging color enhancement and blue laser imaging technology. Dig Endosc. 2014;26 Suppl 1:105-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 26. | Jung SW, Lim KS, Lim JU, Jeon JW, Shin HP, Kim SH, Lee EK, Park JJ, Cha JM, Joo KR, Lee JI. Flexible spectral imaging color enhancement (FICE) is useful to discriminate among non-neoplastic lesion, adenoma, and cancer of stomach. Dig Dis Sci. 2011;56:2879-2886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Ishioka M, Hirasawa T, Tada T. Detecting gastric cancer from video images using convolutional neural networks. Dig Endosc. 2019;31:e34-e35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Yan T, Wong PK, Qin YY. Deep learning for diagnosis of precancerous lesions in upper gastrointestinal endoscopy: A review. World J Gastroenterol. 2021;27:2531-2544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | El Abiad R, Gerke H. Gastric cancer: endoscopic diagnosis and staging. Surg Oncol Clin N Am. 2012;21:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 30. | Huntington CR, Walsh K, Han Y, Salo J, Hill J. National Trends in Utilization of Endoscopic Ultrasound for Gastric Cancer: a SEER-Medicare Study. J Gastrointest Surg. 2016;20:154-63; discussion 163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: a meta-analysis. Gastrointest Endosc. 2011;73:1122-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Okada K, Fujisaki J, Kasuga A, Omae M, Yoshimoto K, Hirasawa T, Ishiyama A, Yamamoto Y, Tsuchida T, Hoshino E, Igarashi M, Takahashi H. Endoscopic ultrasonography is valuable for identifying early gastric cancers meeting expanded-indication criteria for endoscopic submucosal dissection. Surg Endosc. 2011;25:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 289] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 34. | Hasuike N, Ono H, Boku N, Mizusawa J, Takizawa K, Fukuda H, Oda I, Doyama H, Kaneko K, Hori S, Iishi H, Kurokawa Y, Muto M; Gastrointestinal Endoscopy Group of Japan Clinical Oncology Group (JCOG-GIESG). A non-randomized confirmatory trial of an expanded indication for endoscopic submucosal dissection for intestinal-type gastric cancer (cT1a): the Japan Clinical Oncology Group study (JCOG0607). Gastric Cancer. 2018;21:114-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 35. | Takizawa K, Ono H, Hasuike N, Takashima A, Minashi K, Boku N, Kushima R, Katayama H, Ogawa G, Fukuda H, Fujisaki J, Oda I, Yano T, Hori S, Doyama H, Hirasawa K, Yamamoto Y, Ishihara R, Tanabe S, Niwa Y, Nakagawa M, Terashima M, Muto M; Gastrointestinal Endoscopy Group (GIESG) and the Stomach Cancer Study Group (SCSG) of Japan Clinical Oncology Group. A nonrandomized, single-arm confirmatory trial of expanded endoscopic submucosal dissection indication for undifferentiated early gastric cancer: Japan Clinical Oncology Group study (JCOG1009/1010). Gastric Cancer. 2021;24:479-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 36. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1885] [Article Influence: 134.6] [Reference Citation Analysis (0)] |
| 37. | Kim GH, Jung HY. Endoscopic Resection of Gastric Cancer. Gastrointest Endosc Clin N Am. 2021;31:563-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Park CH, Yang DH, Kim JW, Kim JH, Min YW, Lee SH, Bae JH, Chung H, Choi KD, Park JC, Lee H, Kwak MS, Kim B, Lee HJ, Lee HS, Choi M, Park DA, Lee JY, Byeon JS, Park CG, Cho JY, Lee ST, Chun HJ. Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer. Clin Endosc. 2020;53:142-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 39. | Noda M, Kodama T, Atsumi M, Nakajima M, Sawai N, Kashima K, Pignatelli M. Possibilities and limitations of endoscopic resection for early gastric cancer. Endoscopy. 1997;29:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1141] [Article Influence: 47.5] [Reference Citation Analysis (4)] |
| 41. | Esaki M, Ihara E, Gotoda T. Endoscopic instruments and techniques in endoscopic submucosal dissection for early gastric cancer. Expert Rev Gastroenterol Hepatol. 2021;15:1009-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Tao M, Zhou X, Hu M, Pan J. Endoscopic submucosal dissection versus endoscopic mucosal resection for patients with early gastric cancer: a meta-analysis. BMJ Open. 2019;9:e025803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 43. | Kim SG, Ji SM, Lee NR, Park SH, You JH, Choi IJ, Lee WS, Park SJ, Lee JH, Seol SY, Kim JH, Lim CH, Cho JY, Kim GH, Chun HJ, Lee YC, Jung HY, Kim JJ. Quality of Life after Endoscopic Submucosal Dissection for Early Gastric Cancer: A Prospective Multicenter Cohort Study. Gut Liver. 2017;11:87-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 491] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 45. | Choi KS, Jung HY, Choi KD, Lee GH, Song HJ, Kim DH, Lee JH, Kim MY, Kim BS, Oh ST, Yook JH, Jang SJ, Yun SC, Kim SO, Kim JH. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc. 2011;73:942-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 46. | Min JS, Seo KW, Jeong SH. Choice of LECS Procedure for Benign and Malignant Gastric Tumors. J Gastric Cancer. 2021;21:111-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Abe N, Mori T, Takeuchi H, Yoshida T, Ohki A, Ueki H, Yanagida O, Masaki T, Sugiyama M, Atomi Y. Laparoscopic lymph node dissection after endoscopic submucosal dissection: a novel and minimally invasive approach to treating early-stage gastric cancer. Am J Surg. 2005;190:496-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Goto O, Takeuchi H, Kitagawa Y, Yahagi N. Endoscopic Submucosal Dissection (ESD) and Related Techniques as Precursors of "New Notes" Resection Methods for Gastric Neoplasms. Gastrointest Endosc Clin N Am. 2016;26:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1899] [Article Influence: 237.4] [Reference Citation Analysis (1)] |
| 50. | Kim B, Cho SJ. Endoscopic Screening and Surveillance for Gastric Cancer. Gastrointest Endosc Clin N Am. 2021;31:489-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, O'Connor A, Pereira C, Pimentel-Nunes P, Correia R, Ensari A, Dumonceau JM, Machado JC, Macedo G, Malfertheiner P, Matysiak-Budnik T, Megraud F, Miki K, O'Morain C, Peek RM, Ponchon T, Ristimaki A, Rembacken B, Carneiro F, Kuipers EJ; European Society of Gastrointestinal Endoscopy; European Helicobacter Study Group; European Society of Pathology; Sociedade Portuguesa de Endoscopia Digestiva. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012;44:74-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 475] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 52. | Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer. 2013;132:1272-1276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 53. | Yamada H, Ikegami M, Shimoda T, Takagi N, Maruyama M. Long-term follow-up study of gastric adenoma/dysplasia. Endoscopy. 2004;36:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 560] [Article Influence: 32.9] [Reference Citation Analysis (0)] |